Company History
As a Leading Company in the Silicone Industry
Our history started from a discovery
In the 1940s, the founder of the company Nobuhisa Kawaguchi, who was a naval engineering officer, discovered grease-like silicone used as insulation material for electric parts in the engine of the wreckage of a U.S. Grumman fighter plane during World War II. After the war, the company found applications in the medical field by examining various applications while focusing on the potential of the material.



Developed the world's first silicone catheter "Three-Hole Open-Ended Catheter."

Developed the world's first silicone gauze "TREX (non-stick silicone gauze)."


Ohyama Chemical Corporation, predecessor of Fuji Systems, was founded. Started research on and manufacturing of modified epoxy resins. Started research on and manufacturing and sales of the liquid elastomer product Viscol.
Started full scale development of a domestically produced silicone artificial heart with the University of Tokyo.


Established the secondary epoxy resin manufacturer "Japan Pelnox, Ltd." as a joint venture with Nippon Sheet Glass Co., Ltd. Isolated the epoxy resin department.
Changed the company name to "Fuji Systems Corporation." Relocated the head office to Ebisu, Shibuya-ku, Tokyo.

Succeeded in the development of a special membrane (artificial gill) by taking advantage of its permeability to gases. Completed the prototyping of an underwater gas exchange device, and started research on artificial lungs.


Started full scale manufacturing of silicone rubber medical devices under the trademark "PHYCON" with the formal approval of the Ministry of Health and Welfare (currently the Ministry of Health, Labour and Welfare).
Started the manufacturing of the artificial kidney membrane "Nosé - Kiil Envelope" under license from the U.S. Research Corporation.

Signed a general business collaboration agreement with Mitsubishi Corporation, which established its medical division.

Established the Japan Artificial Organ Research Institute (JAOR).
Started the joint development of artificial lungs and membranes with the U.S. Cleveland Clinic.

Signed an agency agreement with Gelman and started the sales of filters.

Signed a domestic agency agreement with Travenol Pacific.

Participated in the space life science experiment program using space shuttles/spacelab with Nagoya University.
Increased the capital to 30 million yen.


Increased the capital to 40 million yen.

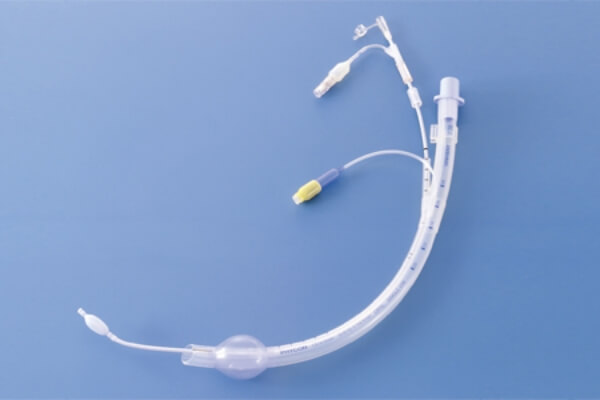
Developed the revolutionary endotracheal tube "UNIVENT" allowing for one-lung ventilation as a joint research project with the Division of Thoracic Surgery, Department of Surgery, Tokai University School of Medicine. The first left upper lung lobectomy was performed on a lung cancer patient using a UNIVENT tube.
Developed Japan's first catheter for self-catheterization "SELFCATH."
Opened the Osaka Branch.

Built the second plant with a clean room in Kamiyabe Industrial Park in Yokohama City.

Developed the open lumen blocker (OLB type) UNIVENT tubes and supplied them to medical institutions around the world.
Built the first new plant in Kamiyabe Industrial Park in Yokohama City.

Shin-Etsu Chemical Co., Ltd. acquired capital in Fuji Systems. Increased the capital to 60 million yen.
Relocated the head office from Ebisu, Shibuya-ku to Yushima, Bunkyo-ku.

Opened the Sapporo Branch.

Received grants for the hollow fiber project from the Tokyo Bureau of Economy, Trade and Industry.
Started the manufacturing and sales of the new composite silicone rubber product "Biotec."
Chairman Nobuhisa Kawaguchi (at the time) was honored by the Governor of Tokyo as a Person of Merit of Tokyo.

Held the 25th anniversary ceremony.

Signed an overseas distributor agreement for UNIVENT with William Stuart from Vitaid, Canada.
Opened the Fukuoka Branch.

Signed a sole distributor agreement for filters in the physical and chemical science field with Gelman Sciences.


Relocated the head office from Yushima, Bunkyo-ku, to Hongo, Bunkyo-ku.
Increased the capital to 90 million yen.


Completed the Shirakawa Plant in Nishigo-mura, Fukushima Prefecture.

Opened the Nagoya Branch.

Completed the Distribution Center in the premises of the Shirakawa Plant.

Opened the Sendai Branch.

Started a joint research project on artificial lungs with Baylor College of Medicine.

Extended the Shirakawa Plant. Obtained EN 46001 and MDD certification.


Opened the Hiroshima Branch.


Completed the new laboratory in Totsuka-ku, Yokohama City.

Extended the Shirakawa Plant.

Obtained ISO 13485:2003 certification.

"Fuji Systems" was transferred to the pure holding company "Fuji Systems HD" as a result of a spin-off incorporation-type company split. The new "Fuji Systems" was created as an operating company.


Obtained an FDA approval for the CELLO Balloon Guide Catheter.

Held the 50th anniversary ceremony.

Started operation of the new Shirakawa Plant.
Opened the Omiya Branch.

Obtained ISO 13485:2016 certification.
Started a joint research project on artificial lungs with the University of Tokyo.

Obtained MDSAP certification.


Opened Kansai Distribution Center in Miki City, Hyogo Prefecture.

receives CE Mark under MDR
- HOME
- Company History





